Intended to assist medical and dental offices with the recommend steps for instrument reprocessing. Use as a handout or poster. Users should refer to published standards and CDC guidelines for additional information.
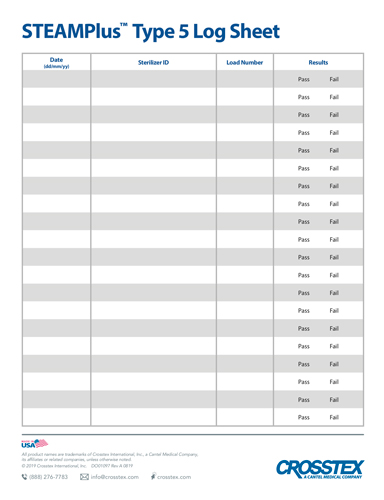
PDFKeep track of sterilization procedures to protect your practice & your patients. Biological monitoring, conducted at least weekly in the US, is key to a successful sterility assurance and risk management program. Please check state recommendations for additional information. Visit: cdc.gov or aami.org. 4 Pages, print and keep in binder for tangible records when proof of testing is required.
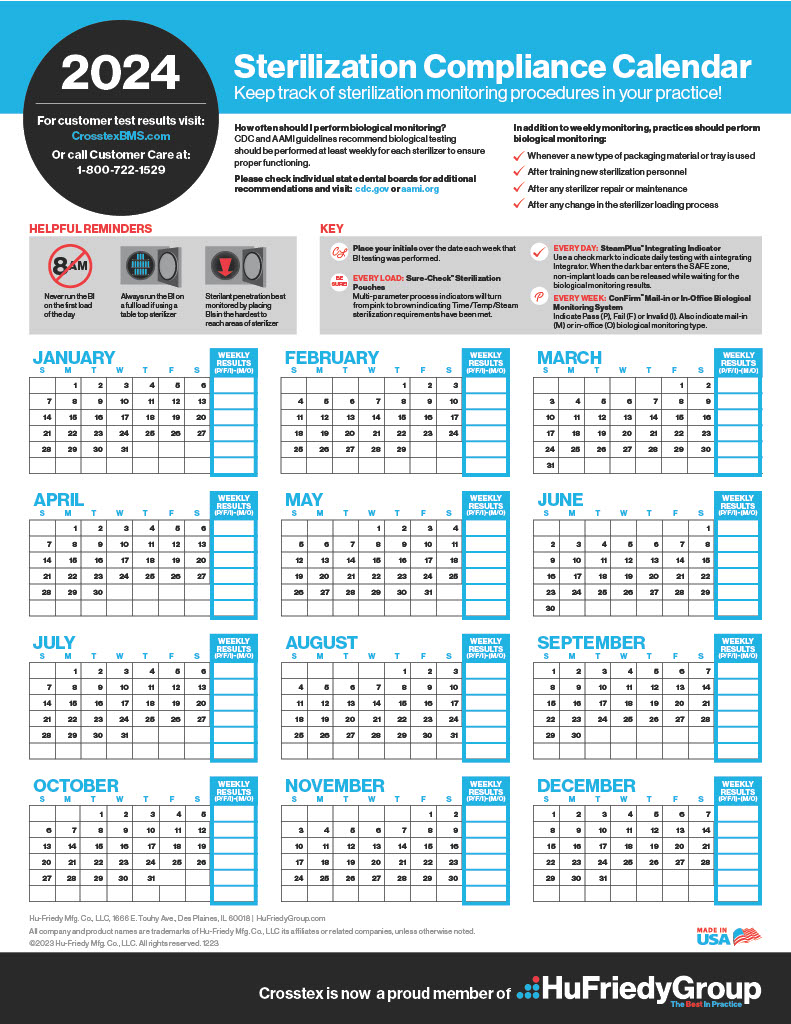
PDFKeep track of sterilization procedures to protect your practice & your patients. Biological monitoring, conducted at least weekly in the US, is key to a successful sterility assurance and risk management program.Please check state recommendations for additional information. Visit: cdc.gov or aami.org. Use as tangible records when proof of testing is required. Format developed for use as hanging in-practice wall chart.
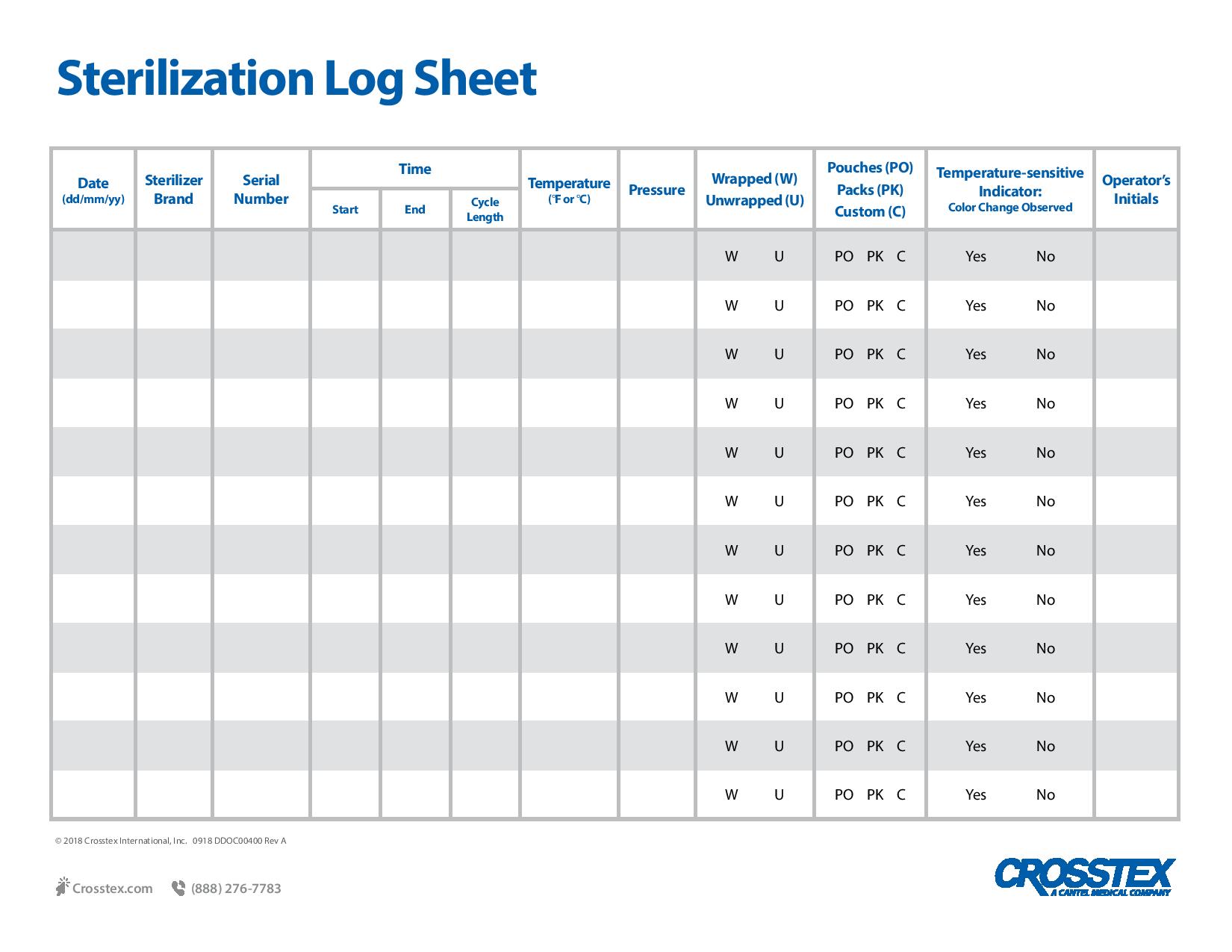
PDFWritten documentation is key to providing proof of sterilization efficacy and provides evidence of testing in the event of an audit. In the case of in-office biological monitoring using the ConFirm 10 or 24 hour product, recommended record keeping includes a log of the sterilization date, the staff member who conducted the procedure, incubation times and the final results for both the test and control vials. Biological monitoring, conducted at least weekly in the US, is key to a successful sterility assurance and risk management program.
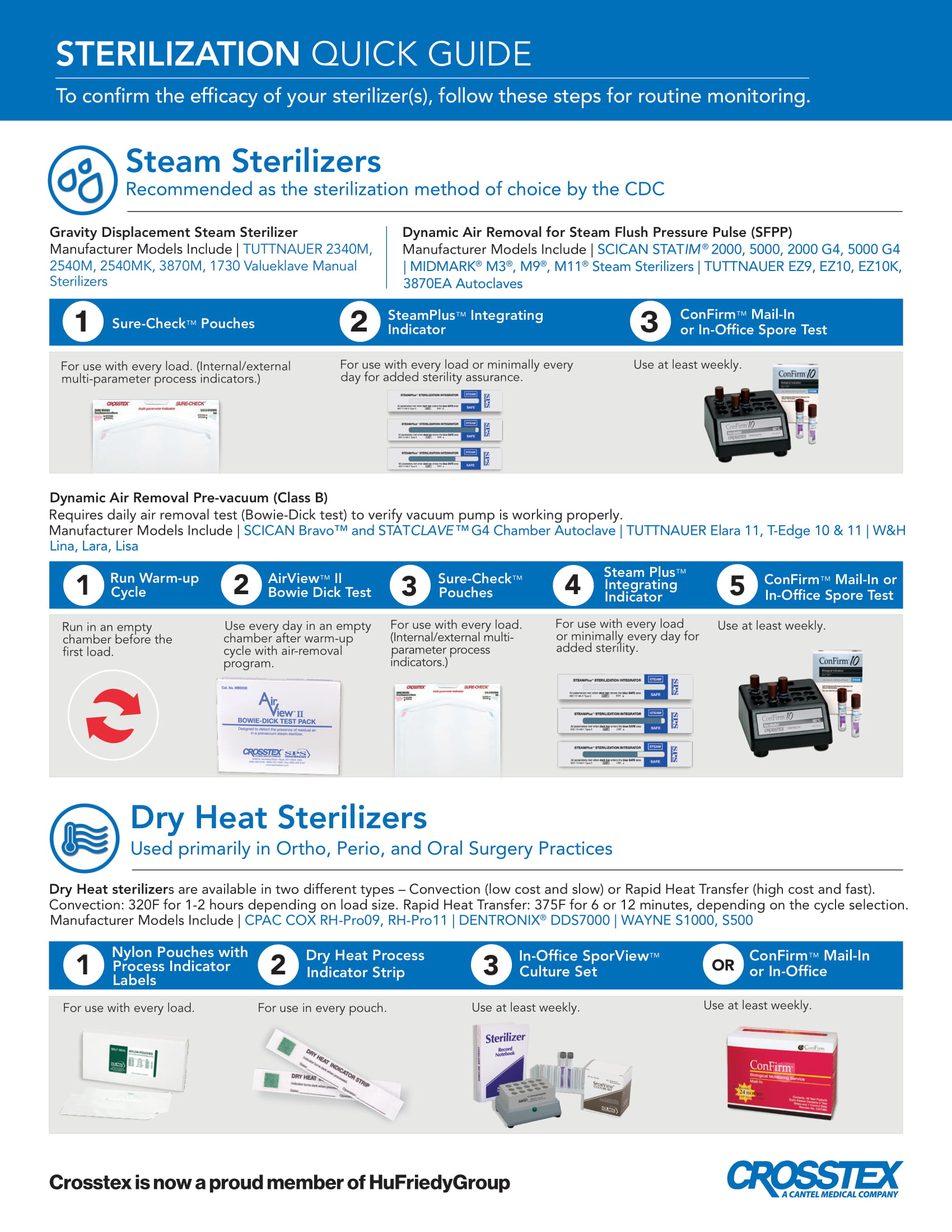
PDFA helpful Crosstex product selection guide that allows you to select the appropriate chemical and biological monitoring products based upon your sterilizer (steam, dry heat, chemical vapor). Includes helpful reminders and handy product codes.
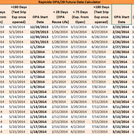
PDFA Future Date Calculator auto-calculates in order to easily allow you to track reuse life and expiration dates of your reusable ortho-phthalaldehyde disinfectant; specially developed for use with Rapicide® OPA/28 High-Level Disinfectant utilized with heat-sensitive, semi-critical devices that are unsuitable for sterilization. Visit OPA28.com for more information on Rapicide® OPA/28 HLD.
XLSXHigh-Level Disinfection Solution Log Sheet to keep track of MRC test results, test dates and operators. Allows for comments, strip lot information, QC test date, QC Test Results and more.
NOTE: Do not use Rapicide OPA/28 High-Level Disinfectant beyond its stated use & reuse life (28 days) even if MRC level is met. Use test strips within 180 days of opening bottle. Visit OPA28.com for more information on Rapicide® OPA/28 HLD.
PDFRapicide(R) OPA/28 Solution 7-Step Wall Poster for medical devices.
PDF